IHumite: Identification of Human Metabolites, an Integrated Prediction based Approach
Drug Metabolite Profiling FDA/ICH MIST require that all “>10%” human plasma metabolites are characterised as early as possible with coverage checked in tox species. Traditional in vitro (incomplete biotransformation) and/or “cold compound First-in-Man” studies followed by LC-MS (operator dependent profiling) risk missing major human metabolites.
The low predictive value of early human metabolite ID results in serious risk of exceeding timelines due to new metabolite findings in phase 3 14C studies. For timely results with your compounds a cost effective and efficient workflow for the identification of human metabolites in plasma has been developed: iHumite
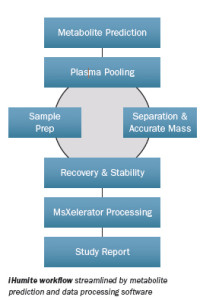
iHumite workflow consists of:
- Phase 1: In silico drug metabolite prediction
- Phase 2: Placebo controlled First-in-Man study: Plasma pooling
- Phase 3: Sample prep and accurate MS measurements
- Phase 4: Data Processing and reporting
Contact MsMetrix for more information.